We bring our patients decades of experience in scientific research. We apply honest medical judgment, accurate diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply it with honest medical judgment, accurate diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply it with sound medical judgment, accurate diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply it with honest medical judgment, proper diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply honest medical judgment, accurate diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply it with honest medical judgment, accurate diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply it with sound medical judgment, accurate diagnoses, and precise prescriptions.
We bring our patients decades of experience in scientific research. We apply it with honest medical judgment, proper diagnoses, and precise prescriptions.
News
Featured
All NEWS
Why choose us
- Medical excellence
- Specialized team
- Leaders in research
- Personalized care
Excellence in medical care and honesty in a team involved in top-level international research

Highly specialized professionals with clinical experience and human quality

We lead internationally recognized research.
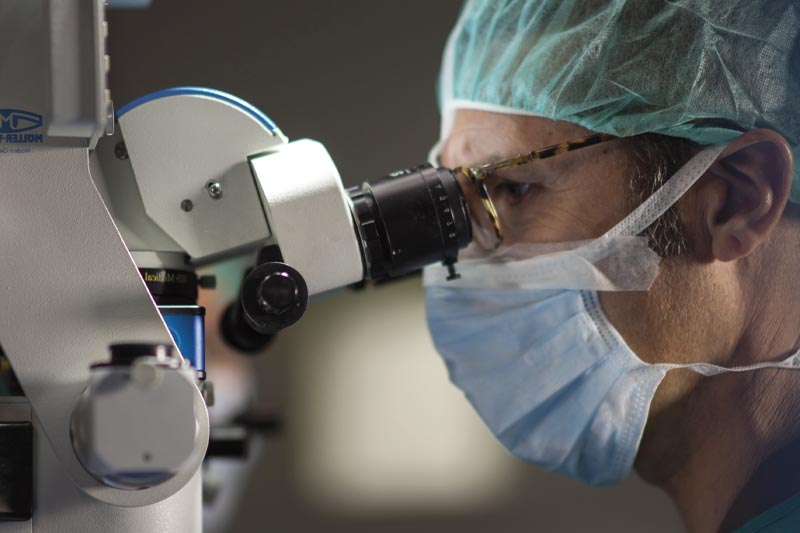
We study each case individually, without preconceived notions or any interest other than the patient’s well-being.

We want you to keep seeing the good things in life.
Because beauty lies in the details, and it’s worth enjoying every one of them
We put all our effort into stopping vision loss. Our vocation is to achieve a future without blindness and to ensure that our patients benefit as soon as possible from the results of the most advanced research.
Most commonly consulted pathologies
Most commonly consulted pathologies
Our commitments
Vocation, connection with patients and families, scientific rigor, and honesty in prescriptions.
Our diagnoses and treatments are based on scientific evidence, and we always put the person first, with no other interest or goal than their physical and emotional well-being.
MEDICAL EXCELLENCE
It is the standard by which we select the professionals who are part of our team.
PERSONALIZED CARE
We steer clear of predefined treatments. We always strive to find the best option for each individual.
RESEARCH
Our scientific vocation leads us to collaborate with the best institutions in the world.
Our commitments
Vocation, connection with patients and families, scientific rigor, and honesty in prescriptions.
Our diagnoses and treatments are based on scientific evidence, and we always put the person first, with no other interest or goal than their physical and emotional well-being.


